Reversible Male Contraceptive Vasalgel Raises $85K for Clinical Trials
A new, no-scalpel vasectomy may arrive if human testing proves successful.
Thanks to $85,000 in donations to the Parsemus Foundation, clinical trials for a new male contraceptive called Vasalgel may be on track to start later this year. The foundation has raised two-thirds of the funding needed to produce a batch of the gel for testing in humans.
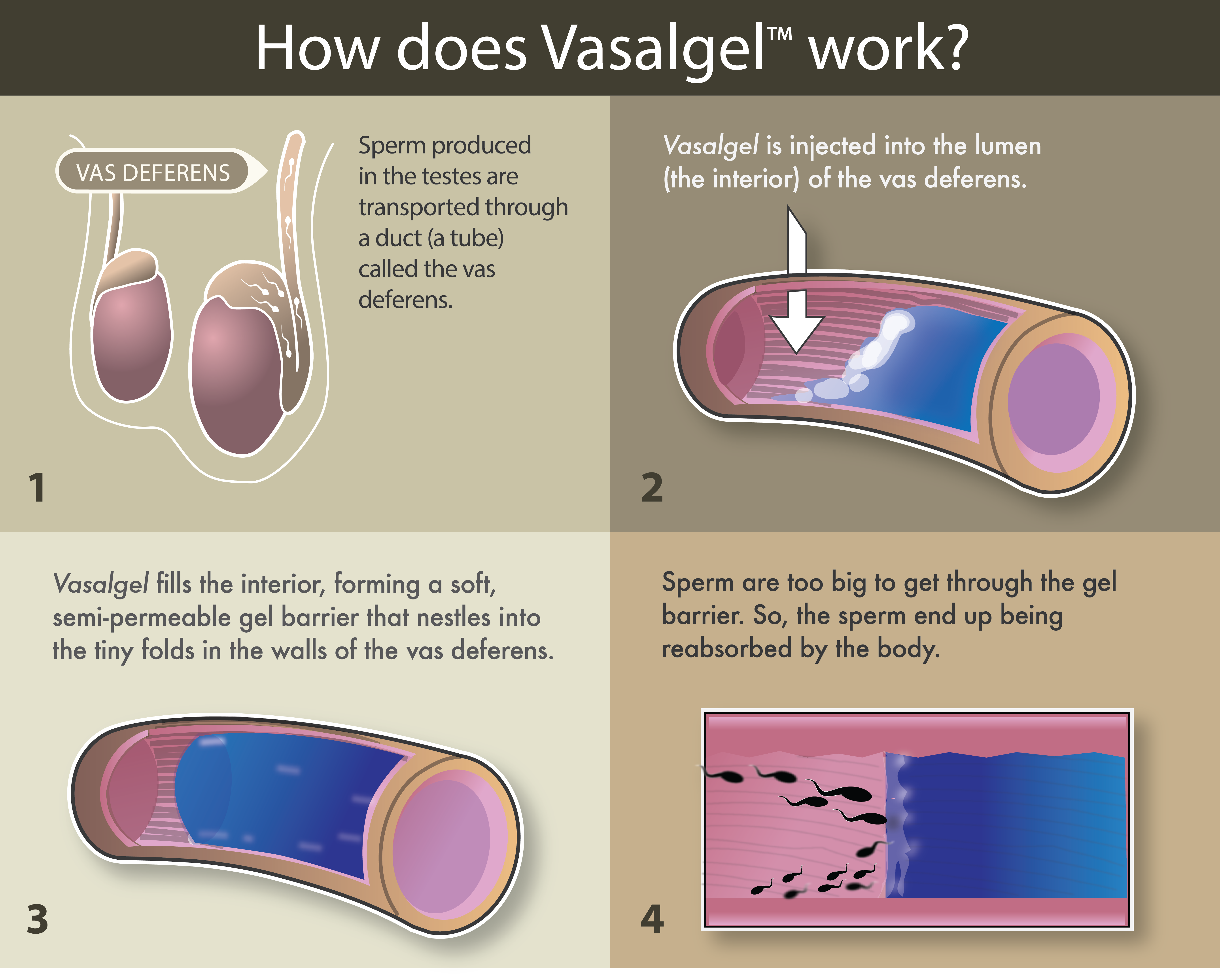
Vasalgel, developed by the Parsemus Foundation, is a nonhormonal male contraceptive to be used in a potentially scalpel-free and reversible vasectomy.
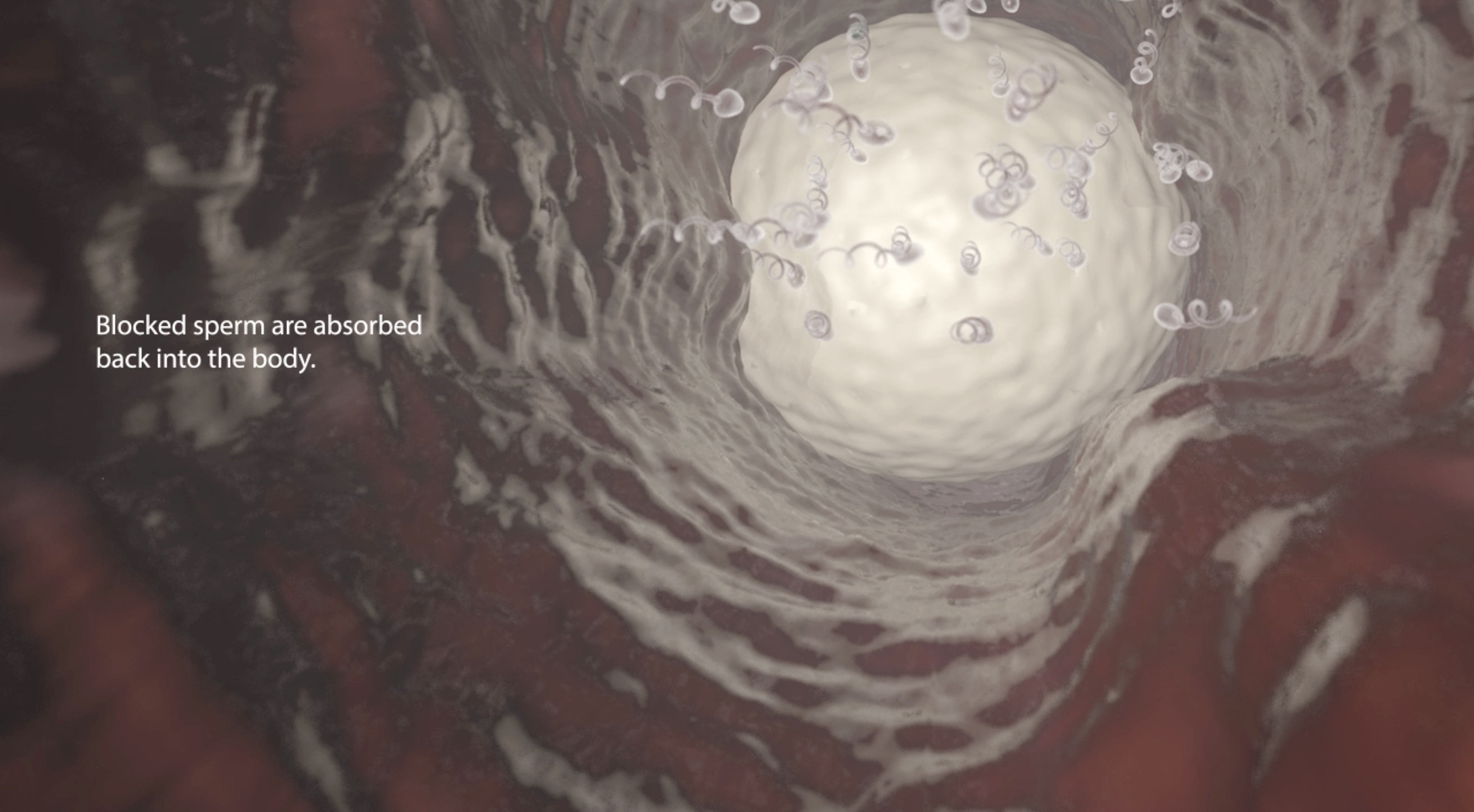
Rather than cutting the vas deferens (the tubes sperm travel through from the testes), as a doctor would in a traditional vasectomy, the procedure involves injecting a gel into the vas deferens. The gel prevents sperm from travelling through the vas ducts, but still allows for other liquids to pass through.
Thus far, the product has been tested in rabbits, but is yet to be used in larger animals and humans.
No longer breeding like rabbits
Two versions of Vasalgel were tested on 12 rabbits. Eleven of the rabbits had virtually no traceable sperm in their semen as early as 29 days post-injection. One rabbit had low doses of sperm in its semen, which was later reduced to zero as well. Both formulas of Vasalgel were equally effective. The rabbits’ azoospermic state (when there are no levels of measurable sperm in semen) lasted the duration of the 12-month study period.
“Results from our study in rabbits were even better than expected. Vasalgel produces a very rapid contraceptive effect which lasted throughout the study due to its unique hydrogel properties. These features are important considerations for a contraceptive product to be used in humans,” said Dr. Donald Waller of Prelabs, LLC. Waller is also a professor of pharmacology and toxicology at the University of Illinois at Chicago .
How can it be reversed?
To reverse the effects of Vasalgel, a sodium bicarbonate (baking soda) solution is injected into the vas deferens to flush out the Vasalgel. This process was tested on seven rabbits, whose sperm flow rapidly restored. Although this test was deemed successful, the Parsemus Foundation is less confident about the same results in larger animals and/or humans.
However, Vasalgel is meant to be a temporary, yet effective, contraception that can ultimately be reversed in men who desire to have children.
The Parsemus Foundation conducted a survey with people who subscribe to Vasalgel updates. Of the 4,467 respondents, 87.3% of male respondents said they’d see Vasalgel as a better alternative to vasectomies, even if it turns out not to be reliably reversible. The survey revealed that many of the respondents did not deem the reversibility as a deal breaker for using Vasalgel.
When can we use it?
The Parsemus Foundation is planning for clinical trials, which would include a small number of men, to begin in late 2016. Afterward, the foundation expects to continue trials on a larger group of men in 2017. The goal is to bring Vasalgel to the market in 2018 and for it to include the reversible aspect.
Low production costs and affordability for all men across the world is another goal for the Parsemus Foundation. But first, the foundation must raise enough funds to secure the first round of trials in humans.
“Contraceptive development is a hugely expensive project. But this is not just another early-stage lead; we’re so close on this one. It’s time to finish the job we’ve started,” Elaine Lissner, Parsemus Foundation Executive Director, said in a press release.
Image source: Parsemus Foundation
Leave a reply
You must be logged in to post a comment.